Electrochemically Enhanced Liquid-phase Catalysis for Simultaneous Purification of Phosphine, Hydrogen Sulfide and Hydrogen Cyanide: Interactions and Synergistic Mechanisms
电化学增强的液相催化作用同时净化磷、硫化氢和氰化氢:相互作用及协同机制
Graphical Abstract
主要内容
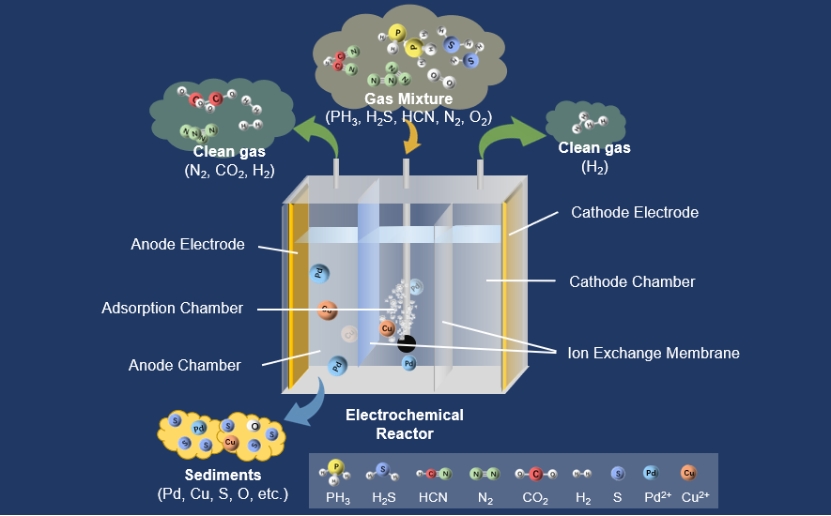
本研究提出了一种简单而有效的电化学强化液相催化方法,用于同时去除工业尾气中的PH3、H2S和HCN。使用Pd(II)和Cu(II)作为液相催化剂。在电化学反应和液相催化的协同作用下,PH3、H2S和HCN最终被氧化成PO43-、单质硫、SO42-、CO2和N2。电化学过程中产生的HO-、H2O2和HO2-具有很强的氧化能力。研究了催化剂类型、电化学条件(电极电压、电极电流等)、接入顺序和单/双组份催化剂等影响参数。详细分析了净化过程中PH3、H2S和HCN组分之间的相互作用规律。在3V的恒定电压下,使用Pd/Cu催化剂时达到了最佳净化效果,PH3、H2S和HCN的净化率约为84%、100%和95%。此外,在模拟去除尾气中的PH3、H2S和HCN时,该系统更加稳定。本研究提出了一种同时去除有毒有害工业废气的实用策略。它大大减少了有害废气的排放,并使工业过程更加清洁。
In this research, a simple and effective electrochemically enhanced liquid-phase catalysis approach was proposed for simultaneous removal of PH3, H2S, and HCN from industrial tail gases. Pd(II) and Cu(II) were used as the liquid-phase catalyst. Under the synergistic effect of electrochemical reactions and liquid-phase catalysis, PH3, H2S, and HCN were finally oxidized to PO43-, elemental sulfur, SO42-, CO2, and N2. HO·, H2O2, and HO2· generated in the electrochemical process had a strong oxidative ability. Influencing parameters such as the catalyst types, electrochemical conditions (electrode voltage, electrode current, etc.), access sequence and mono/bi-component catalyst were investigated. The law of interaction among PH3, H2S and HCN components in the purification process was analyzed in detail. At a constant voltage of 3 V, the optimal purification effect was achieved when Pd/Cu catalyst was used, PH3, H2S and HCN purification rates were approximately 84%, 100% and 95%. Furthermore, the system is more stable when mimicking the removal of PH3, H2S, and HCN from tail gas. This study proposes a practical strategy for removing poisonous and hazardous industrial waste gases at the same time. It significantly reduces hazardous exhaust emissions and enables cleaner industrial processes.
Highlight
1. 提出了电化学增强型液相催化技术。
The electrochemical enhanced liquid-phase catalysis was proposed.
2. 同步高效脱除PH3, H2S和HCN。
Simultaneous removal PH3, H2S and HCN with high efficiency.
3. 详细分析了PH3, H2S和HCN同步净化机制。
The synchronous purification mechanism was analyzed in detail.
4. 详细分析了PH3、H2S和HCN之间的协同/抑制净化效果。
The synergistic / inhibitory purification effect between PH3, H2S and HCN was analyzed.
Mechanism Analysis
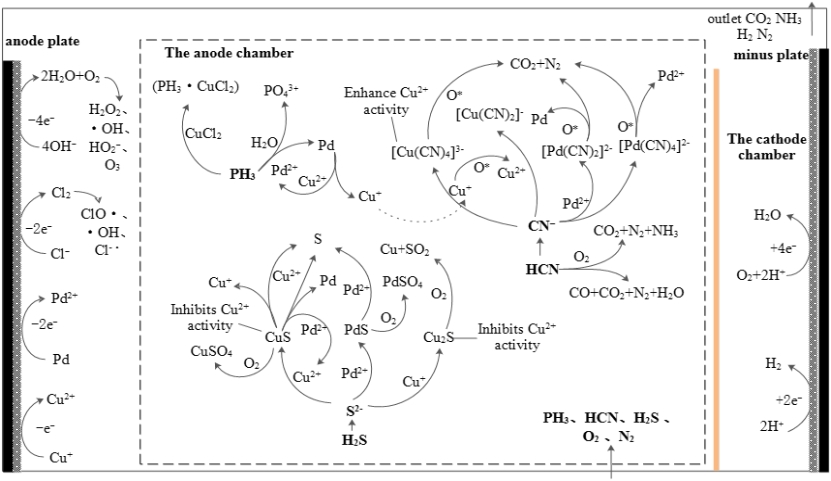
在多污染气体同步净化的过程中,PH3、H2S和HCN之间存在着明显的交互作用。具体来说,H2S阻碍了PH3的净化,而HCN则促进了H2S的净化。PH3的氧化被Pd2+转化为Pd0的过程所促进,但Pd2+容易与H2S解离产生的S2-结合,形成PdS沉淀。Pd2+和S2-的结合降低了体系中自由有效氧化成分的含量,抑制了PH3的氧化。形成的硫化物或多硫化物在气流扰动和吸收室的扩散迁移作用下进入阳极附近。然后它们被阳极产生的活性物质氧化,形成单体S并释放出过渡金属。
在电液共净化过程中,在液相催化剂的作用下,进入反应体系的所有PH3、一部分H2S和一部分HCN分别被氧化为H3PO4、S、CO2和N2(NH3)。一些HCN和一些H2S与自由金属离子形成络合物[Mc(CN)n]2-n和硫化物McSx。在气流扰动、扩散迁移和电场力的共同作用下,这些络合物、硫化物和在与液相催化剂反应中产生的固体颗粒Mc0移动到阳极。在阳极产生的活性氧氧化了[Mc(CN)n]2-n。配体CN-被氧化成CO2和N2(或NH3、NO3-)。释放出的中心离子被氧化成更高的价态。McSx被氧化成单质S(部分进一步氧化成SO42-),金属离子被释放。固体颗粒Mc0被氧化成高价离子Mc2+。被电极氧化恢复活力的金属离子通过阳离子膜或其下部迁移到吸收室,参与后续反应。吸收室中产生的CO2、N2和阴极室中通过电解产生的H2从阴极室的气体出口排出。在阳极室中通过氧化和电解产生的CO2、N2、O2、H2和少量的CO从阳极室的气体出口排出。
In the process of simultaneous purification, there were interactions among PH3, H2S and HCN. Specifically, H2S hindered the purification of PH3, and HCN promoted on H2S purification. The oxidation of PH3 was facilitated by the conversion process of Pd2+ to Pd0, but Pd2+ was prone to combine with S2- generated by the dissociation of H2S to form PdS precipitate. The combination of Pd2+ and S2- reduced the content of free effective oxidation components in the system and inhibited the oxidation of PH3. The sulfides or polysulfides formed entered the vicinity of the anode under the effect of airflow perturbation and diffusion migration in the absorption chamber. They were then oxidized by active substances produced at the anode to form monomeric S and release transition metals.
In the electro-hydraulic co-purification process, all PH3, a part of H2S and a part of HCN that entered the reaction system were oxidized to H3PO4, S, CO2 and N2 (NH3), respectively, in the presence of a liquid-phase catalyst. Some HCN and some H2S formed complexes [Mc(CN)n]2-n and sulfides McSx with free metal ions. These complexes, sulfides, and solid particles Mc0 produced in the reaction with the liquid-phase catalyst moved to the anode under the combined effect of airflow perturbation, diffusion migration and electric field forces. Reactive oxygen produced at the anode oxidized [Mc(CN)n]2-n. The ligand CN- was oxidized to CO2 and N2 (or NH3, NO3-). Released central ions were oxidized to a higher valence state. McSx was oxidized to singlet S (partly further oxidized to SO42-), and metal ions were released. Solid particles Mc0 were oxidized to higher valence ions Mc2+. Metal ions rejuvenated by electrode oxidation migrated through the cation membrane or its lower part to the absorption chamber to participate in subsequent reactions. CO2, N2 produced in the absorption chamber and H2 produced by electrolysis in the cathode chamber were discharged from the gas outlet of the cathode chamber. CO2, N2, O2, H2 and a small amount of CO produced by oxidation and electrolysis in the anode chamber were discharged from the gas outlet of the anode chamber.
文章链接:Electrochemically Enhanced Liquid-phase Catalysis for Simultaneous Purification of Phosphine, Hydrogen Sulfide and Hydrogen Cyanide: Interactions and Synergistic Mechanisms - ScienceDirect
